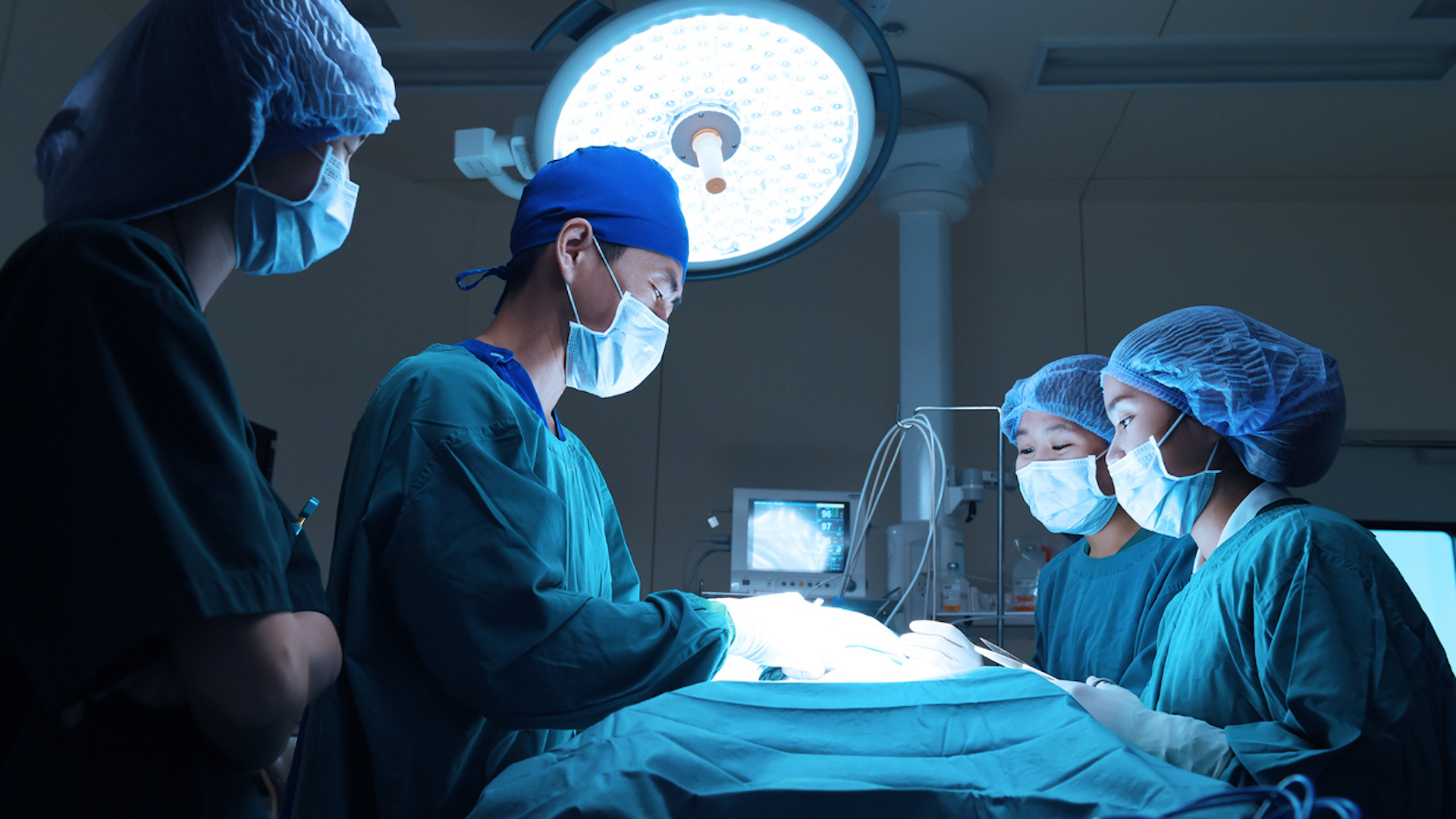
About 10 million patients undergo inpatient surgical procedures per year, accounting for roughly 25% of all hospital stays.1 The most common operations range from cesarean sections and joint replacements, to hip fracture repair and neurosurgical or intra-abdominal procedures. Each carries its own unique needs, but they all share a commonality — the risk of operating room contamination and resulting SSIs.
While the Centers for Disease Control and Prevention (CDC) confirms most of the infections are treatable with antibiotics, it also points to SSIs as a leading cause of hospital readmissions.1 Neither outcome is desirable for patients or healthcare facilities, which makes minimizing operating room contamination a key ongoing focus.
The Role of Surgical Draping in OR Contamination Mitigation
The challenge presented by OR contamination is multifaceted. The surgical site itself must be protected, but threats abound throughout the operating room if sterile sites aren’t maintained.
Surgical draping is the first line of defense. Used in conjunction with recommended best practices, products designed expressly to protect patient surgical areas and clinicians, back tables, and equipment create barriers to microbial migration.
Limiting the handling and repositioning of surgical draping is critical. The tops of operating and prep area tables are considered sterile. Surgical draping that hangs downward from the edges of the tables is nonsterile. Indiscriminate folding, handling, or repositioning can inadvertently introduce portions of nonsterile draping into the sterile field — resulting in contamination.
Further, surgeon and operating team gowns and gloves are sterile. Draping that is prematurely placed or handled multiple times poses a greater threat to contaminate the team’s coverings. This is especially serious in the case of hands, as inappropriate hand preparation practices or other hand contamination are among the leading causes of SSIs.2
Given the role surgical draping plays in OR contamination prevention, the quality and convenience of the products used are paramount.
From patient drapes to table and equipment covers, TIDI offers the brands that support caregivers and protect patients. Learn more about Sterile-Z®, C-Armor®, and TIDIShield® surgical draping and covering options.
SOURCES
1PS Net, Surgical Site Infections | PSNet, September 2019
2 Infection Control Today, Fighting Surgical Site Infections, Undated